Many patients have sent Dysautonomia International questions about the association between antiphospholipid syndrome and POTS, after an article appeared in the medical journal Lupus on this topic on February 25, 2014. Dysautonomia International asked the first author of this article, Dr. Jill Schofield, to address some of the questions raised by the patient community in the following blog post. Please note that this is not meant to replace advice given by your own physician.
What Dysautonomia Patients Should Know About Antiphospholipid Syndrome
by Jill R. Schofield, MD
We have recently published the first clinical association of postural orthostatic tachycardia syndrome (POTS),
What is APS?
APS is a complex autoimmune disorder that is associated with several different antiphospholipid antibodies. These antibodies may be directed against clotting factors, platelets, and/or the cells that line blood vessel walls and they cause the blood to be too sticky. This results in an increased risk of blood clots in:
1) Arteries–causing most commonly stroke or heart attack.
2) Veins–causing deep vein thrombosis (DVT) of the legs and/or pulmonary embolus (PE) of the lungs.
3) Placenta–causing recurrent mi
In addition to an increased risk for blood clots, a number of other manifestations may occur in APS due to “sludging” of the blood. The list of these non-clotting manifestations is long and they are less well known to most physicians. Some of these manifestations include migraine (which may be severe and refractory to usual treatments), memory loss, seizures and stress fractures. We have now demonstrated that POTS, NCS and OH may also occur as non-clotting manifestations of APS.
How is APS diagnosed?
The Sapporo criteria for the diagnosis of definite APS requires:
1. Clinical criteria: Thrombosis (blood clot) or very specific pregnancy complications (such as three or more miscarriages).
2. Laboratory criteria: Medium to high titer antiphospholipid antibodies on more than one occasion at least 12 weeks apart.
These criteria were designed for rigorous clinical research studies, not for diagnosis. Unfortunately, most practicing physicians believe they were designed for diagnosis and this has resulted in patients with low titer antibodies and/or non-clotting manifestations not being diagnosed with APS, when they do have the syndrome. My hope is that we can change this perception, because I believe that with earlier diagnosis, we can prevent the thrombotic events!
For our study, we used the following criteria:
At least one clinical manifestation of the syndrome (including the non-clotting manifestations) along with the presence of one or more of the antiphospholipid antibodies in any titer:
1) Anticardiolipin IgG and/or IgM
2) Beta 2 glycoprotein I IgG and/or IgM
3) Lupus anticoagulant
It is common to have only one of these antibodies, but some patients have two or even all three. Occasionally various infections might cause a transient elevation in one or more of these tests, so the diagnostic criteria require you to have one or more of these antibodies on more than one occasion at least 12 weeks apart. Notably, many of the patients in our study had low titers of the APS antibodies.
The lupus anticoagulant test has a misleading name because it is not a test for lupus and it is associated with increased clotting, not decreased clotting as the name implies. APS, however, may occur along with lupus, as well as Sjogrens syndrome or rheumatoid arthritis. It may also occur on its own. Most APS experts are either rheumatologists, hematologists or obstetricians, but most physicians are familiar with these tests, they can be ordered at any lab and they are relatively inexpensive.
Once a diagnosis of APS is made, there is no indication to repeat the antibody tests. APS is not known to just resolve, but the antibodies are known to wax and wane over time. There are many stories of patients who have had their levels fall into the normal range when their physicians repeatedly tested their antibodies and when told they no longer had APS and could stop their blood thinners, they went on to develop stroke or other major clotting events.
Regarding imaging tests, CT or MRI scans are used to test for stroke or other blood clots in APS patients with suspicious symptoms and clots in this syndrome can be found in any blood vessel. Some APS patients have “white spots” on brain MRI scans; these are felt to represent very tiny clots. Ultrasound is commonly used to test for blood clots in the legs when there is new leg pain and/or swelling.
How common is APS?
APS is not rare. It has been estimated to affect approximately 1 out of 100 people (1% of the population). It is, however, underdiagnosed.
We do not know how many POTS, NCS or OH patients have APS, but Dysautonomia International recently funded a research project designed by Dr. Svetlana Blitshteyn to try to shed some light on the topic of autoimmune markers and autoimmune conditions in patients with POTS. Dysautonomia International will make an announcement when Dr. Blitshetyn’s study results are released.
We also do not yet know how often autonomic disorders occur in APS patients.
Should all patients with POTS be tested for APS?
Because this is a newly described clinical association, we have a lot to learn. At this time, I believe all POTS patients should be tested for APS; other physicians might disagree. At the very least, I believe all POTS patients with any of the following should be tested for APS: migraine, memory loss, balance trouble, livedo reticularis, Raynaud’s phenomenon, history of miscarriage, another autoimmune condition, a family history of blood clots or a family history of autoimmune disease. These were the most common findings in the patients in our study. Also, of note, three of the 15 APS patients included our study also had Joint Hypermobility Syndrome (JHS).
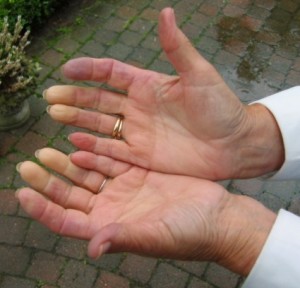
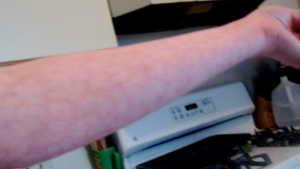
Raynaud’s Phenomenon Livedo Reticularis
The reason I believe all POTS patients without an apparent cause should be tested for APS is that POTS caused by APS might improve with a trial of aspirin, clopidogrel, heparin, warfarin and/or IVIG. Many of these agents increase the risk of bleeding, however, which makes many physicians not experienced with APS nervous about using them in APS patients who have not had a clotting episode. Because APS is a very hypercoagulable condition, however, APS patients (even those on high doses of blood thinners) have a much greater risk of clotting than bleeding. Despite this, Professor Hughes believes that many APS patients have been under-treated due to physician concerns about bleeding.
Additionally, APS is a serious medical condition and early diagnosis can help reduce the risk of major complications. If you have one or more of the APS antibodies, you are at an increased risk for blood clots. If you are aware you have one or more of these antibodies, you can reduce your risk of blood clots by avoiding cigarettes, birth control pills or hormone replacement therapy. You can also be sure that if you have other vascular risk factors, such as high blood pressure, high cholesterol or diabetes, they are treated aggressively. Aspirin has also been shown to reduce the risk of arterial events in patients with APS antibodies. Additionally, Professor Yehuda Shoenfeld’s research has shown that vitamin D levels are low (less than 15 ng/ml) in half of patients with APS and that low levels are associated with an increased risk for clotting and non-clotting manifestations of the syndrome. So it makes sense for these patients to also be treated with vitamin D.
How is APS treated?
In addition to the points made above, APS patients who have had an arterial or venous clotting event are generally treated with blood thinners (usually warfarin or heparin) for life. There are also several newer oral anticoagulants, one of which is presently being studied in APS patients in London. Until this data is available, these drugs are generally not recommended for most APS patients. APS patients with recurrent miscarriages are treated with aspirin and usually heparin (warfarin is contraindicated in pregnancy) throughout their pregnancy and for at least 6 weeks after delivery.
Importantly, Professor Hughes has found over the years that many of the non-clotting manifestations of APS often improve significantly or may even be completely aborted with anti-platelet agents such as aspirin or clopidogrel, and/or warfarin or heparin. He has also found this to be true for autonomic symptoms in some APS patients. Two patients in our study with POTS that did not improve with standard APS treatments (despite improvement of other APS manifestations) improved significantly with regular intravenous immunoglobulin (IVIG) therapy. Unfortunately, IVIG is very expensive and many insurance companies require more data than two case reports before approving its use for a specific indication.
Where can you find additional resources on APS?
Dysautonomia International has a brief explanation of APS on its website, as well as some links to APS related journal articles and non-profit organizations. You can find a physician experienced in APS by going to APSAction.org, an international organization started in 2010 to improve collaboration amongst APS experts and to facilitate APS research. An excellent patient forum on APS is HealthUnlocked Hughes syndrome and Professor Hughes has written a great book for patients entitled, Understanding Hughes Syndrome: Case Studies for Patients.
 Dr. Schofield is a Johns Hopkins trained internist who has developed an interest in APS over the last few years. She currently practices in Denver, Colorado but plans to develop a multi-disciplinary (i.e. involving physicians from many specialties) APS clinic in an academic environment and is currently exploring options for where best to do this.
Dr. Schofield is a Johns Hopkins trained internist who has developed an interest in APS over the last few years. She currently practices in Denver, Colorado but plans to develop a multi-disciplinary (i.e. involving physicians from many specialties) APS clinic in an academic environment and is currently exploring options for where best to do this.