Researchers around the world have started studying the use of plasma from people who have recovered from COVID-19 to treat those who are severely ill. Blood banks are also asking the public to donate blood, because many blood drives have been cancelled. Many of you have asked if it’s safe for people with POTS or neurocardiogenic syncope to donate blood or plasma, so we checked in with our Medical Advisory Board for their input.
What is convalescent plasma?
For over a century, doctors have used convalescent plasma to treat serious infections. Plasma is collected from a volunteer who has recently recovered from an infection. Their plasma usually contains a lot of antibodies generated by their immune system to fight off the infection. Their plasma is then infused into someone who is still sick and needs help fighting off the same infection.
Is convalescent plasma a proven treatment for COVID-19?
According to the US Food & Drug Administration (FDA), “[a]lthough promising, convalescent plasma has not yet been shown to be effective in COVID-19.” Since there are no known effective treatments, multiple options are being studied. There is some evidence suggesting that convalescent plasma may help some COVID-19 patients, especially those who are seriously ill. This is why the FDA recently announced an effort to study this treatment option. Other countries are also planning convalescent plasma studies.
Is it safe for people with POTS to donate plasma for COVID-19 research?
Prior research studies have found that a majority of people with POTS have a lower than normal volume of blood, also known as hypovolemia. However, not all POTS patients had this problem, and for some it is just slightly below normal volume.
The decision on whether or not a POTS patient should donate blood or plasma should be made on a case by case basis with input from your doctor. There is nothing about POTS that makes it inherently dangerous to donate blood or plasma. You may feel temporarily more lightheaded, which can also happen to healthy people when they donate blood or plasma.
If you do decide to donate blood or plasma, you should increase your oral fluids before and after your donation. Ask the donation center if you can receive 1L of normal saline after your donation. It may also help to be in a reclined position when donating blood or plasma. Most blood/plasma donation centers have recliner chairs available.
Is it safe for people with neurocardiogenic syncope (also known as vasovagal syncope) to donate plasma for COVID-19 research?
Yes, just like POTS, the decision on whether or not an NCS patient should donate blood or plasma should be made on a case by case basis with input from your doctor. There is nothing about NCS that makes it inherently dangerous to donate blood or plasma. You may feel temporarily more lightheaded, which can also happen to healthy people when they donate blood or plasma. For some individuals with NCS/VVS, the sight of blood or needles may trigger a syncopal event.
If you do decide to donate blood or plasma, you should increase your oral fluids before and after your donation. Ask the donation center if you can receive 1L of normal saline after your donation. It may also help to be in a reclined position when donating blood or plasma. Most blood/plasma donation centers have recliner chairs available.
Where can I find out how to donate convalescent plasma?
In the US, if you have recovered from confirmed COVID-19, visit https://ccpp19.org/donors to find out how to register to donate plasma. Canada is also putting together a study, which you can read about here. In other countries, we recommend contacting your local academic medical center, Red Cross, or local blood donation clinic to ask if they are collecting plasma from individuals who have recovered from COVID-19.
Where can I obtain plasma for someone who has severe COVID-19?
This research-based treatment is only being used in hospital settings for severely ill patients. If you are asking on behalf of a friend or loved one who is severely ill in the hospital with confirmed COVID-19, ask the hospital if they are part of a convalescent plasma research trial.
In the US, clinicians can register their hospital to be part of the national trial here.
If a US hospital is not part of an approved clinical trial, the FDA can also give physicians emergency authorization to use convalescent plasma in a single patient by telephone. This is called a Single Patient Emergency Investigational New Drug approval.
Several other countries, including Canada and the UK, are in the process of setting up COVID-19 convalescent plasma research studies. Ask the hospital if they are using convalescent plasma for their severe COVID-19 patients.
Where can I learn more about COVID-19 and how it impacts people with dysautonomia and related conditions?
Visit our Coronavirus Information Page: dysautonomiainternational.org/coronavirus.
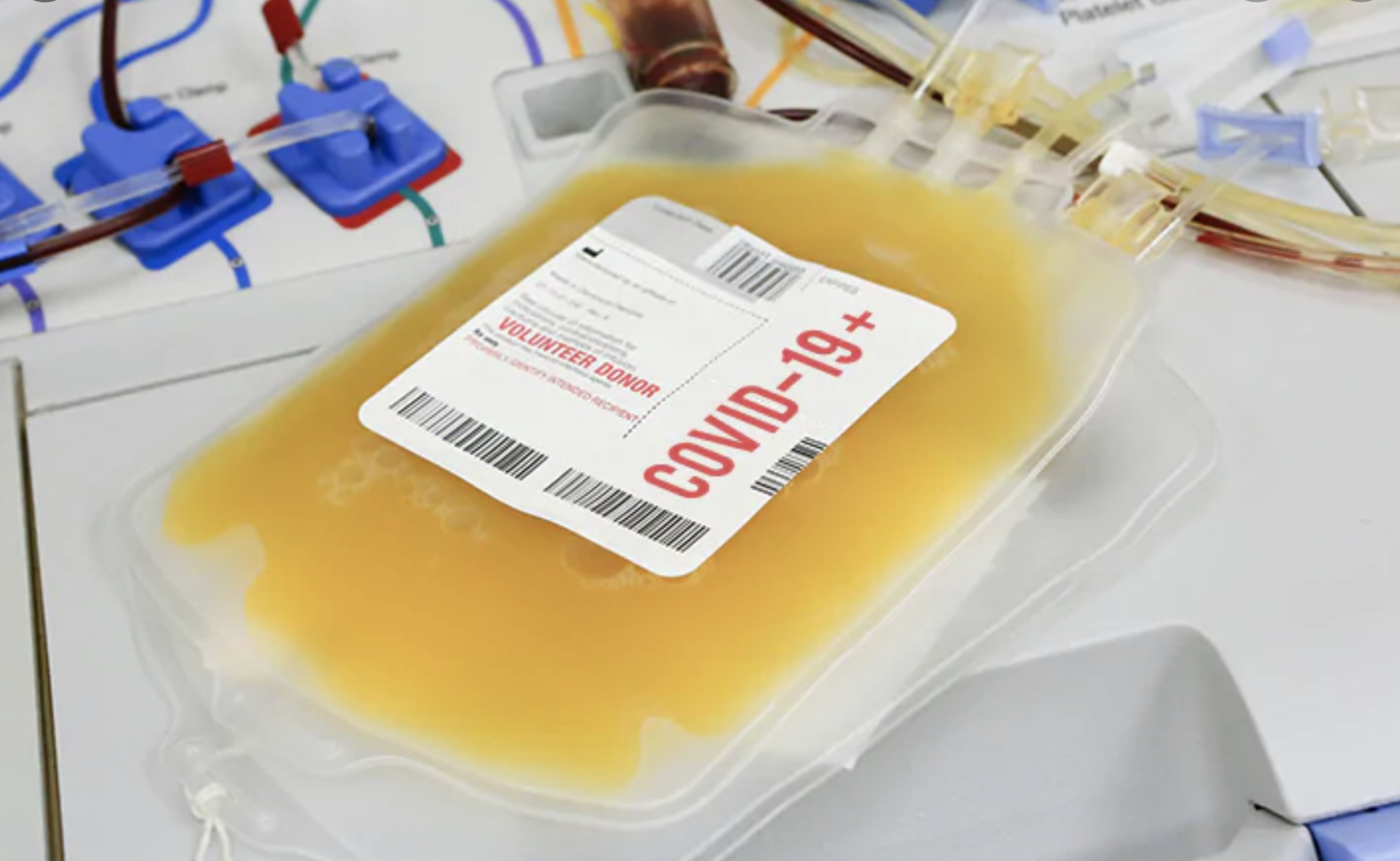 Photo from MedPageToday’s story on COVID-19 plasma research.
Photo from MedPageToday’s story on COVID-19 plasma research.
Stay up to date on the latest dysautonomia news, research and events by joining Dysautonomia International’s email list.